E – 1477 Enthalpy, Entropy, Mollier Diagram and Steam Table
$200.00
Courses Included
In this course, you will get introduced to units, concepts, terms, principles, laws and equations pertaining to two of the most fundamental entities in thermodynamics: enthalpy and entropy.
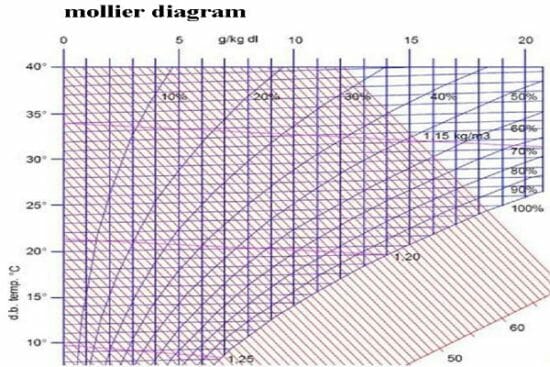
You will get introduced to Mollier diagram and learn how to utilize it in the assessment of enthalpy and entropy changes as temperature and pressure are changed.
Understanding steam tables and navigating through them is an essential skill associated with thermodynamics. You will get an opportunity to review and learn about the various parameters tabulated in saturated and superheated steam tables; how those parameters interact and change as a function of each other. The difference between saturated steam tables and superheated steam tables is highlighted. The significance of – and the association between – saturated pressure and saturation temperature is covered. The significance and operation of interpolation and double
interpolation – as it pertains to the use of steam tables – is described. The review and application of steam tables is bolstered through examples and case studies.
This course caters to engineers of all disciplines, as well as technicians, facilities managers and executives who are not intimately familiar with thermodynamics principles and practices. The course is based on the text titled “Thermodynamics Made Simple for Energy Engineers,” by S. Bobby Rauf, Fairmont Press, 2010.